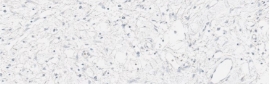
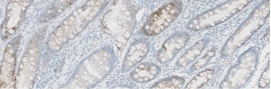
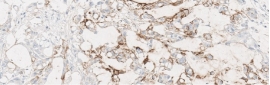
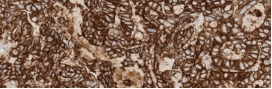
Testing for CLDN18.2 uses standard IHC staining methods, with interpretation according to membranous stain intensity and percentage of tumor cells stained.1
Testing for CLDN18.2 uses standard IHC staining methods, with interpretation according to membranous stain intensity and percentage of tumor cells stained.1,2